Translating Time
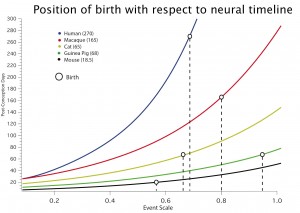
One of the principal ways an adult phenotype might be altered by evolution is to change the timing and relative duration of organizing processes in development. We have progressively organized a database starting from early events in neurogenesis in common laboratory species (Finlay and Darlington, 1995), then extending it to a wider range of neuroembryological processes (Clancy et al. 2000, Clancy et al., 2001) and species (Darlington, Dunlop and Finlay, 1999), and finally extending it to include neural and behavioral events corresponding to the first three years of human postnatal development in 18 species (Workman et al., 2013; Finlay and Workman 2013).The sequence and relative duration of neurodevelopmental events is highly conserved in mammals, though absolute duration varies extremely, ranging from around 20 days in some rodents to 200 or more in primates for initial neurogenesis. The order in which structures cease neurogenesis has direct consequences for brain scaling: structures generating stem cells latest in development are the ones which become the largest in large brains, like the cortex and cerebellum (Finlay, Darlington and Nicastro, 2001). There are only a few cases so far in which developmental sequences are altered to change brain organization, including relative delay of neocortical generation in carnivores and primates, producing a relatively larger neocortex (Reep et al., 2007) and relative delay of retinogenesis in large-eyed nocturnal mammals (Dyer et al, 2009). Brain development can be shifted quite dramatically with respect to somatic maturational events: in the adjacent graph, from Workman et al., 2013, the progress of 5 species as measured in days (Y axis) against a common maturational scale, the”event scale” (X axis) can be seen, with the dropped lines showing the relative position of birth in the different species.Current work is extending the modelling to later postnatal neural and behavioral events in more species, such as eye growth, postnatal neurogenesis and details of myelination, in a still greater range of species, particularly including primates. A more ambitious goal, organized by Ph.D. student Ryutaro Uchiyama, is to relate the detailed knowledge we now have of brain development to changing life history patterns in humans compared to primates and mammals more generally (e.g. “Situating social learning in an evolutionarily conserved program of mammalian brain development”).
The Translating Time Website This evolutionarily-motivated work has direct consequences for biomedical research. In order to time research investigations or medical interventions accurately, a rational method of determining the equivalent maturational state of the brain across species would be of obvious use. The Translating Time site, periodically updated, will do the math to identify the day of a particular neurodevelopmental event in any one of our 18 modeled species, or simply identify the respective days in two species for any maturational state. We invite any researcher to contribute developmental data to this evolving enterprise!
Bibliography
Workman, A., Charvet, C., Clancy, B., Darlington, R.B. and Finlay, B.L. (2013) Modeling transformations of neurodevelopmental sequences across mammalian species. Journal of Neuroscience 17: 7368-7383 doi: 10.1523/jneurosci.5746-12.2013 (Faculty of 1000 Recommendation, May, 2013)
Finlay, B.L. and Workman, A.J. (2013) Human exceptionalism. Trends in Cognitive Sciences 17: 199-201 doi: 10.1016/j.tics.2013.03.001 (Faculty of 1000 Recommendation, May, 2013)
Finlay, B.L. and Clancy, B. (2008) Chronology of the development of the mouse visual system. Eye, Retina and Visual System of the Mouse L. Chalupa and R.W. Williams, eds. MIT Press. pp 257-265.
Reep, R., Darlington, R.B. and Finlay, B.L. (2007) The limbic system in mammalian brain evolution. Brain, Behavior and Evolution 70: 57-70
Clancy, B., Kersh, B, Hyde, J.,. Anand, K.J.S, Darlington, R.B. and Finlay, B.L. (2007) Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 5: 79-94
Clancy, B., Finlay, B.L., Darlington, R.B. and Anand, K.J.S. (2007) Extrapolating brain development from experimental species to humans. Neurotoxicology 28: 931-937
Finlay, B.L., Darlington, R.D. and Nicastro, N. (2001) Developmental structure of brain evolution. Behavioral and Brain Sciences 24: 263-308 (with commentary and response)
Franco, E.C.S., Finlay, B.L.,Silveira, L.C.L., Yamada, Y.C, and Crowley, J.C. (2001) Conservation of absolute foveal area in New World primates: A constraint on eye size and conformation. Brain, Behavior and Evolution 56: 276-286
Clancy, B., Darlington, R.B. and Finlay, B.L. (2001) Translating developmental time across mammalian species. Neuroscience 105: 7-17
Clancy, B.E. and Finlay, B.L. (2001) Neural correlates of early language learning. In M. Tomasello and E. Bates (Eds.) Essential Readings in Language Development.
Clancy, B., Darlington, R.B. and Finlay, B.L. (2000) The course of human events: predicting the timing of primate neural development. Developmental Science 3: 57-66
Darlington, R.B., Dunlop, S.A. and Finlay, B.L. (1999) Commentary: Neural development in metatherian and eutherian mammals:variation and constraint. Journal of Comparative Neurology 411: 359-368
Finlay, B.L., Hersman, M.N. and Darlington, R.B. (1998) Patterns of vertebrate neurogenesis and the paths of vertebrate evolution. Brain, Behavior and Evolution 52: 232-242
Finlay, B.L. and Darlington, R.B. (1995) Linked regularities in the development and evolution of mammalian brains. Science 268: 1578-1584
Evolution of the Visual System, focusing on New World Primates
The visual periphery of vertebrates is highly variable, with adaptations for nocturnal and diurnal vision, ranges of color perception, specialized central versus panoramic vision and multiple styles of head and eye movements, and is thus an ideal candidate for an evo-devo approach, to understand how development may be altered to produce these specializations, and to compare mechanisms of central versus peripheral nervous system change. In a long-term collaboration with Luiz Carlos de Lima Silveira and colleagues, we are investigating such changes in New World monkeys.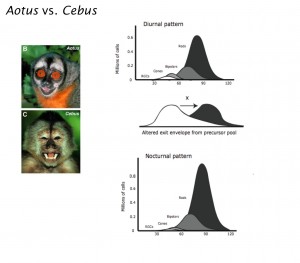
The first step in this extensive project was the characterization of numerical differences in the number of identified cell classes in the retina and central nervous system of a variety of New World primates, including species of marmosets, tamarins, squirrel monkeys, capuchins, howler monkeys and owl monkeys, as well as description of features of gross morphology, such as axial length of the eye and the organization of the fovea. These included the numbers of rods, cones, rod bipolar cells and ganglion cells, with special attention to the unusual retina of the howler monkey. Using stereological techniques, we studied the scaling of the pulvinar complex, the lateral geniculate nucleus and the cortex, comparing neuron counts and volumetric and areal assessments in the case of the cortex.
With this information in hand, we examined how alterations in development might have been called on over evolutionary time to produce these specializations. Earlier work examined how retinal ganglion cells and central targets might adjust themselves to changing convergence ratios. We proposed organizational theories of how extensions in developmental schedules might automatically link eye size to cone versus rod numerical scaling requirements, how the organization of the fovea might change in response to changing cell complements. We tested, and partially proved the hypotheses that a length of the relative duration of photoreceptor and neuron genesis with respect to the schedule of cell specification in the owl monkey, Aotus, versus the capuchin monkey, Cebus apella, might be the single change needed to produce a multitude of adaptations to nocturnality.Current work integrates the quite different set of allometric alterations in the visual system of birds in comparison with mammals, with particular interest in midbrain versus thalamocortical systems.
Bibiography
Troilo, D.B., Xiong, M., Crowley, J.C. and Finlay, B.L. (1996) Factors controlling the dendritic arborization of retinal ganglion cells. Visual Neuroscience 13: 721-734Xiong, M. and Finlay, B.L. (1996) What do developmental mapping rules optimize? Progress in Brain Research 112: 350-361
Silveira, L.C.L., Yamada, E. S., Franco, E.C.S. and Finlay, B. L. (2000) The specialization of the owl monkey retina for night vision. Colour Research and Application 26: 118-122
Franco, E.C.S., Finlay, B.L., Silveira, L.C.L., Yamada, Y.C, and Crowley, J.C. (2001) Conservation of absolute foveal area in New World primates: A constraint on eye size and conformation. Brain, Behavior and Evolution 56: 276-286
Kaskan, P., Franco, C., Yamada, E., Silveira, L.C.L., Darlington, R. and Finlay, B.L (2005) Peripheral variability and central constancy in mammalian visual system evolution. Proceedings of the Royal Society: Biological Sciences 272: 91-100
Finlay, B. L., Silveira, L.C.L. and Reichenbach, A. (2005) Comparative aspects of visual system development. In: The Structure, Function and Evolution of the Primate Visual System J. Kremers, ed. John Wiley and Sons pp. 37-72
Chalfin, B.P., Cheung, D.T., Muniz, J.A.P.C., Silveira, L.C.L. and Finlay, B.L. (2007) Scaling of neuron number and volume of the pulvinar complex in New World primates: comparisons with humans, other primates and mammals.Journal of Comparative Neurology 504: 265-274
Finlay, B.L. (2008) The developing and evolving retina: using time to organize form . Brain Research 1192: 5-16
Finlay, B.L.,Franco, E.C.S., Yamada, E.S., Crowley, J.C., Parsons, M.E.,, Muniz, J.A. P.C and L.C.L. Silveira (2008) Number and topography of cones, rods and optic nerve axons in New and Old World primates: implications for the evolution of retinal development. Visual Neuroscience 25: 289-299.
Dyer, M.A. , Martins, R., da Silva Filho, M., Muniz, J.A.,Silveira, L.C.L., Cepko, C. and Finlay, B.L. (2009) Developmental sources of conservation and variation in the evolution of the primate eye. Proceedings of the National Academy of Sciences, USA. 106: 8963 – 8968
Lameirão, V.O.C.,1, Hamassaki, D.E., Rodrigues, A.R, de Lima, S.M, Finlay, B.L. and Silveira, L.C.L. (2009) Rod bipolar cells in the retina of the capuchin monkey (Cebus apella): Characterization and distribution. Visual Neuroscience 26: 389–396
Finlay, B.L. and Osorio, D.C. (2011) Introduction to “Comparative, ecological and developmental aspects of visual system design and function. Visual Neuroscience 28
Finlay, B.L., Charvet, C.J., Bastille, I., Cheung, D.T., Muniz, J.A.P.C., and L.C.L.Silveira (2014). Scaling the primate lateral geniculate nucleus: Niche and neurodevelopment in regulation of magnocellular and parvocellular cell number and nucleus volume. Journal of Comparative Neurology 522:1839-1857 doi: 10.1002/cne.23505
Muniz, J. A. C. P. de Athaide, L.M., Gomes, B.D., Finlay, B.L., Silveira, LCL (2014) Ganglion cell and displaced amacrine cell density distribution in the retina of the howler monkey (Alouatta caraya). PLoS ONE 9(12): e115291. doi:10.1371/journal.pone.0115291
Bastille, I, Charvet, C.J. and Finlay, B.L. Avian versus mammalian patterns in visual system evolution. In preparation.
Cheung, D.T., Muniz, J.A.P.C., Crowley, J.C., Parsons, M.E., Yamada, E., Chalfin, B.P., Silveira, L.C.L. and Finlay B.L. Scaling the visual system: placing primate retina and brain organization in its mammalian and vertebrate context. In preparation.
Evolution of the cortex in its developmental context
Much of the early work of this laboratory was focused on the developmental neurobiology of the cortex
Bibliography
Finlay, B.L. and Brodsky, P.B. (2006) Cortical evolution as the expression of a program for disproportionate growth and the proliferation of areas.. Evolution of Nervous Systems J.H. Kaas, ed. Elsevier: Oxford university Press pp. 73-96
Finlay, B.L. (2004) The Calvinist cortex: penetrating evolutionary predestination. Commentary on “Cortex, countercurrent context, and dimensional integration of lifetime memory” by B. Merker. Cortex 40: 577-579
Kingsbury, M. A. and Finlay, B. L. (2001) The cortex in multidimensional space: where do cortical areas come from? Commentary Article. Developmental Science 4: 125-156
Anderson, M.L., Finlay, B.L. (2013). Allocating structure to function: the strong links between neuroplasticity and natural selection. Frontiers in Human Neuroscience 7 doi: 10.3389/fnhum.2013.00918 Faculty of 1000 recommendation , August, 2014
Syal, S. and Finlay, B.L. (2011) Thinking outside the cortex: Social motivation in the evolution and development of language. Developmental Science 14: 417-430
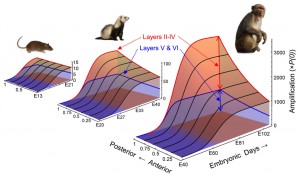
Cahalane DJ, Charvet CJ and Finlay BL (2012). Systematic, balancing gradients in neuron density and number across the primate isocortex. Frontiers in Neuroanatomy 6:28.
Charvet, C.J., Cahalane, D.J. and Finlay, B.L. (2014) Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cerebral Cortex 25: 147-160 doi:10.1093/cercor/bht214
Charvet, C.J. and Finlay, B.L (2014) Evo-devo and the primate isocortex: the central organizing role of intrinsic gradients of neurogenesis. Karger Symposium “The Problem of Human Brain Evolution: Integrating Diverse Approaches” Brain, Behavior and Evolution 84:81-92 doi: 10.1159/000365181
Cahalane, D.J., Charvet, C.J., and Finlay, B.L. (2014) Modeling local and cross-species variations in the cerebral cortex as arising from a common mechanism. Proceedings of the National Academy of Sciences, USA 111: 17642-17647 doi/10.1073/pnas.1409271111
Finlay, B.L. and Uchiyama, R. (2015) Developmental mechanisms channeling cortical evolution. Trends in Neurosciences 38:69-76
Charvet, C.J, Reep, R.L. and Finlay, B.L. (2015) Evolution of cytoarchitectural landscapes in the mammalian isocortex: Sirenians (Trichechus manatus), carnivores, rodents and primates. Journal of Comparative Neurology